India Business Information
Corona News
Visitors: 15865
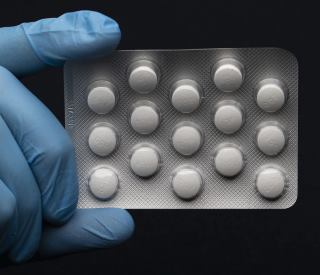
US : FDA authorizes widespread use of unproven drugs to treat coronavirus
March 31, 2020
The Food and Drug Administration has given emergency approval to US Government, plan to distribute millions of doses of anti-malarial drugs to hospitals across the US, saying it is worth the risk of trying unproven treatments to slow the progression of the disease caused by the novel coronavirus in seriously ill patients.
The FDA’s emergency authorization does not cover longer-term use of the drugs to prevent the coronavirus infection, although doctors have been prescribed the drugs “off label'' for weeks in response to the pandemic.
Long-term use of the drugs also is associated with a chance of developing a form of vision loss called retinopathy, but the use of the drugs to fight virus in an infected patient is only for a few days.
Popular Post(s)...
/Sports
/Online Shopping
/Online Shopping
/Online Shopping
/Sports
Menu
- IPL
- World University rankings 2022, 2023
- Weight Loss
- Website Monetization
- United States Free Business Directory
- Tech Support
- Study in US
- Study in United Kingdom
- Study in India
- Sports
- Pets Name
- Movie and Theatres
- London Business Directory
- Aadhaar Services
- Indian Food Recipes
- Home
- American Food Recipes
- Astrology
- Best Hits Of Madhuri Dixit
- Bollywood Celebrities
- Business India
- Corona News
- DTDC Courier Tracking
- Ecom Express Tracking
- Fully Funded UK Scholarships
- Hit Video Songs
- India Track Your Courier
- States-and-Capitals
- Tamilnadu-Business
- Currency Exchange
- Online Shopping
- Baby Names by Birth Star
 ( 5 ) by 1 User(s).
( 5 ) by 1 User(s).